Envelope trafficking and signaling
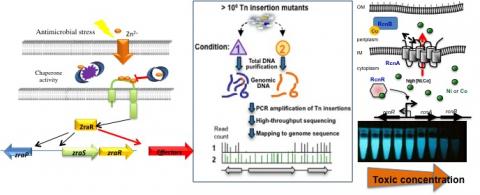
The role of metals at the bacteria / host interface
A transcriptome of D. dadantii in Arabidopsis showed that many metal transporters are severely repressed in the early hours of infection. A Zur mutant was shown to be disadvantaged during plant infection in the Tn-seq analysis that was carried on in the lab (Royet et al, 2019). Zur is known to control Zn homeostasis in some bacteria, however Zur regulates virulence functions in Xanthomonas campestris, G3E GTPases in Agrobacterium tumefaciens and an endopeptidase in Vibrio cholerae. Finally, our recent studies highlighted the function of Zn as an alarm of membrane damages (Petit-Hartlein et al., 2015, Rome et al., 2018). This new concept needs further investigations. Our current project is to study the role of Zn, Zur and metal transport in the virulence of D. dadantii. A growing number of publications highlight the crucial role of trace metals in host-bacteria interaction. However, many of these studies focus on iron and currently few on other metals. For that purpose, a proteomic approach will be undertaken (with the help of the IBCP MS facility), and candidate proteins will be further investigated at the regulatory and functional levels. Implication G. Condemine, E. Guéguen and A. Rodrigue
Structure-functional studies of D. dadantii type 2 secretion system, T2SS
Gram-negative bacteria employ T2SS to deliver toxins and hydrolases to the external environment and host tissues. D. dadantii secretes an array of pectinases and some other proteins through a T2SS. The assembly, organization and dynamics of this unique but widespread molecular machine remains elusive and are in the focus of our ongoing and scheduled studies. Recently we have undertaken a large comparative analysis to unravel the structure and function of the pilus within the T2SS machine. This study (ANR SYNERGY_T2SS started in 2019) includes three French teams working on T2SS and structural biology experts in NMR and cryo-EM. We will determine the T2SS pilus structure and characterize its dynamics and interactions with secreted substrates at atomic level. The use of several model pathogens will allow us to develop a unifying model of T2SS.
Recent progress in cryo-electron microscopy allowed an enormous breakthrough in structural analysis of whole multiprotein cell machineries. We have undertaken an EM study of the whole T2SS of D. dadantii, a multi-protein complex spanning the whole bacterial envelope. Our team provides a biological expertise, functional analysis and site-specific in vivo photo-crosslinking to unravel relevant protein-protein interfaces within the T2SS complex. Implication V. Shevchik and A. Rodrigue
A new old mechanism of protein covalent attachment to peptidoglycan
A recent MTSB study showed that one component of D. dadantii T2SS is covalently attached to the peptidoglycan (PG). From the Seventies, in Gram-negative bacteria, only Lpp, also called Braun’s lipoprotein, is known to be covalently linked to the PG thus attaching the PG to the outer membrane. In E. coli, six paralogous L,D-transpeptidases are involved either in the Lpp-PG attachment or in an atypical crosslinking of stem peptides in the PG. The latter mechanism allows bacteria to resist against β-lactam antibiotics. D. dadantii has only four such putative L,D-transpeptidases and we plan to identify their roles in protein-PG attachment, their functional implications for T2SS and resistance to β-lactams. A similar yet enzymatically profoundly different mechanism of protein attachment to the PG is widespread in Gram-positive bacteria and our finding suggests that this phenomenon could also be frequent in Gram-negative bacteria. We will perform a systematic search for other proteins covalently attached to the PG (in Dickeya, Pectobacterium and E. coli) and study the mechanisms and functional relevance of this. Implication V. Shevchik